Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Tachihara, Joji; Takato, Kiyoto; Okita, Takatoshi; Satone, Hiroshi*; Suzuki, Michitaka*
Mechanical Engineering Journal (Internet), 8(3), p.21-00022_1 - 21-00022_9, 2021/06
To reduce the hold-up of the nuclear fuel materials in the glove box and the external exposure dose, the technology of the MOX powder adhesion prevention by the nanoparticle coating to the acrylic panels of the glove box has been developed. The surface analysis by means of atomic force microscopy (AFM) showed that the acrylic test piece surface coated with nanoparticles had a higher root mean square roughness value than that non-coated with nanoparticles. Due to the formation of nano-sized tiny rugged surface, the nanoparticle coating reduced the minimum adhesion force between the UO particles and the acrylic test piece surface with the smallest particle size of about 5
particles and the acrylic test piece surface with the smallest particle size of about 5  m where desorption was observed, by about one-tenth. Moreover, the nanoparticle coating reduced the amount of the MOX powder adhering to the acrylic test piece to about one-tenth. In this study, it was found that applying the nanoparticle coating to the acrylic panels of glove box can prevent the adhesion of nuclear fuel materials. This method is effective for reducing the hold-up of the nuclear fuel materials in the glove box, the external exposure dose and improving the visibility of the acrylic panels.
m where desorption was observed, by about one-tenth. Moreover, the nanoparticle coating reduced the amount of the MOX powder adhering to the acrylic test piece to about one-tenth. In this study, it was found that applying the nanoparticle coating to the acrylic panels of glove box can prevent the adhesion of nuclear fuel materials. This method is effective for reducing the hold-up of the nuclear fuel materials in the glove box, the external exposure dose and improving the visibility of the acrylic panels.
Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Tachihara, Joji; Takato, Kiyoto; Okita, Takatoshi; Satone, Hiroshi*; Suzuki, Michitaka*
Proceedings of 2020 International Conference on Nuclear Engineering (ICONE 2020) (Internet), 6 Pages, 2020/08
To reduce the hold-up of the nuclear fuel materials in the glove box and the external exposure dose, the technology of the MOX powder adhesion prevention by the nanoparticle coating to the acrylic panels of the glove box has been developed. Due to the formation of nano-sized tiny rugged surface, the nanoparticle coating reduced the minimum adhesion force between the UO particles and the acrylic test piece surface with the smallest particle size of about 5
particles and the acrylic test piece surface with the smallest particle size of about 5  m where desorption was observed, by about one-tenth. Moreover, the nanoparticle coating reduced the amount of the MOX powder adhering to the acrylic test piece to about one-tenth. In this study, it was found that applying the nanoparticle coating to the acrylic panels of glove box can prevent the adhesion of nuclear fuel materials. This method is effective for reducing the hold-up of the nuclear fuel materials in the glove box, the external exposure dose and improving the visibility of the acrylic panels.
m where desorption was observed, by about one-tenth. Moreover, the nanoparticle coating reduced the amount of the MOX powder adhering to the acrylic test piece to about one-tenth. In this study, it was found that applying the nanoparticle coating to the acrylic panels of glove box can prevent the adhesion of nuclear fuel materials. This method is effective for reducing the hold-up of the nuclear fuel materials in the glove box, the external exposure dose and improving the visibility of the acrylic panels.
 -radiation and H
-radiation and H O
O induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acid
induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acidKumagai, Yuta; Jonsson, M.*
Dalton Transactions (Internet), 49(6), p.1907 - 1914, 2020/02
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)This study aims to reveal possible involvements of organic acids in the oxidative dissolution of UO . Using phthalic acid as a model compound, we have measured adsorption on UO
. Using phthalic acid as a model compound, we have measured adsorption on UO and investigated effects on the reaction between H
and investigated effects on the reaction between H O
O and UO
and UO and on oxidative dissolution induced by
and on oxidative dissolution induced by  -irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H
-irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H O
O and UO
and UO in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO
in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO surface, it is not involved in the interfacial reaction by H
surface, it is not involved in the interfacial reaction by H O
O . In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H
. In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H O
O generated by radiolysis was consumed by UO
generated by radiolysis was consumed by UO . The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO
. The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO .
.
 O
O concentration on oxidative dissolution of U
concentration on oxidative dissolution of U O
OKumagai, Yuta
Hoshasen Kagaku (Internet), (107), p.77 - 78, 2019/04
Reaction of hydrogen peroxide (H O
O ) with uranium dioxide (UO
) with uranium dioxide (UO ) oxidizes U(IV) to water-soluble U(VI). In the concept of direct geological disposal of spent nuclear fuel, this reaction is expected to induce dissolution of UO
) oxidizes U(IV) to water-soluble U(VI). In the concept of direct geological disposal of spent nuclear fuel, this reaction is expected to induce dissolution of UO matrix of the spent fuel. This study investigate effect of H
matrix of the spent fuel. This study investigate effect of H O
O concentration on the kinetics and the yield of U(VI) dissolution of this reaction. A series of experiments of the reaction of H
concentration on the kinetics and the yield of U(VI) dissolution of this reaction. A series of experiments of the reaction of H O
O with UO
with UO powder dispersed in water has been carried out. The experimental results reveal that increase in the H
powder dispersed in water has been carried out. The experimental results reveal that increase in the H O
O concentration slows down the reaction and decreases the yield of U(VI) dissolution. This observation suggests that a reaction intermediate is generated in the course of the H
concentration slows down the reaction and decreases the yield of U(VI) dissolution. This observation suggests that a reaction intermediate is generated in the course of the H O
O reaction on the surface of UO
reaction on the surface of UO .
.
 induced by H
induced by H O
O and
and  -irradiation
-irradiationKumagai, Yuta; Fidalgo, A. B.*; Jonsson, M.*
Journal of Physical Chemistry C, 123(15), p.9919 - 9925, 2019/04
Times Cited Count:19 Percentile:62.54(Chemistry, Physical)Radiation-induced oxidative dissolution of uranium dioxide (UO ) is one of the most important chemical processes of U driven by redox reactions. We have examined the effect of UO
) is one of the most important chemical processes of U driven by redox reactions. We have examined the effect of UO stoichiometry on the oxidative dissolution of UO
stoichiometry on the oxidative dissolution of UO induced by hydrogen peroxide (H
induced by hydrogen peroxide (H O
O ) and
) and  -ray irradiation. By comparing the reaction kinetics of H
-ray irradiation. By comparing the reaction kinetics of H O
O between stoichiometric UO
between stoichiometric UO and hyper-stoichiometric UO
and hyper-stoichiometric UO , we observed a significant difference in reaction speed and U dissolution kinetics. The stoichiometric UO
, we observed a significant difference in reaction speed and U dissolution kinetics. The stoichiometric UO reacted with H
reacted with H O
O much faster than the hyper-stoichiometric UO
much faster than the hyper-stoichiometric UO . The U dissolution from UO
. The U dissolution from UO was initially much lower than that from UO
was initially much lower than that from UO , but gradually increased as the oxidation by H
, but gradually increased as the oxidation by H O
O proceeded. The
proceeded. The  -ray irradiation induced the U dissolution that is analogous to the kinetics by the exposure to a low concentration (0.2 mM) of H
-ray irradiation induced the U dissolution that is analogous to the kinetics by the exposure to a low concentration (0.2 mM) of H O
O . The exposure to higher H
. The exposure to higher H O
O concentrations caused lower U dissolution and resulted in deviation from the U dissolution behavior by
concentrations caused lower U dissolution and resulted in deviation from the U dissolution behavior by  -ray irradiation.
-ray irradiation.
 O
O and UO
and UO
Fidalgo, A. B.*; Kumagai, Yuta; Jonsson, M.*
Journal of Coordination Chemistry, 71(11-13), p.1799 - 1807, 2018/07
Times Cited Count:29 Percentile:88.5(Chemistry, Inorganic & Nuclear)In this work, we have studied the reaction between H O
O and UO
and UO with particular focus on the nature of the hydroxyl radical formed as an intermediate. Experiments were performed to study the kinetics of the reaction at different initial H
with particular focus on the nature of the hydroxyl radical formed as an intermediate. Experiments were performed to study the kinetics of the reaction at different initial H O
O concentrations. The results show that the consumption rates at a given H
concentrations. The results show that the consumption rates at a given H O
O concentration are different depending on the initial H
concentration are different depending on the initial H O
O concentration. This is attributed to an alteration of the reactive interface, likely caused by blocking of surface sites by oxidized U/surface-bound hydroxyl radicals. The U dissolution yield decreases with increasing initial H
concentration. This is attributed to an alteration of the reactive interface, likely caused by blocking of surface sites by oxidized U/surface-bound hydroxyl radicals. The U dissolution yield decreases with increasing initial H O
O concentration. This is expected from the mechanism of catalytic decomposition of H
concentration. This is expected from the mechanism of catalytic decomposition of H O
O on oxide surfaces. As the experiments were performed in solutions containing 10 mM and a strong concentration dependence was observed in the 0.2 - 2.0 mM H
on oxide surfaces. As the experiments were performed in solutions containing 10 mM and a strong concentration dependence was observed in the 0.2 - 2.0 mM H O
O concentration range, we conclude that the intermediate hydroxyl radical is surface bound rather than free.
concentration range, we conclude that the intermediate hydroxyl radical is surface bound rather than free.
Morita, Koji; Tobita, Yoshiharu; kondo, Satoru; E.A.Fischer*
JNC TN9400 2000-005, 57 Pages, 1999/05
An improved analytic equation-of-state (EOS) model using flexible thermodynamic functions is developed for a reactor safety analysis code, SIMMER-III. The present EOS model is designed to have adequate accuracy in describing thermodynamic properties of reactor-core materials over wide temperature and pressure ranges and to consistently satisfy basic thermodynamic relationships without deterioration of the computing efficiency. The fluid-dynamic algorithm for pressure iteration consistently coupled with the EOS model is also described in the present report. The EOS data of the basic core materials, uranium dioxide, mixed-oxide fuel, stainless steel, and sodium, are developed up to the critical point by compiling the most up-to-date and reliable sources using basic thermodynamic relationships. The thermodynamic consistency and accuracy of the evaluated EOS data are also discussed by comparison with the available sources.
Morita, Koji; Tobita, Yoshiharu; kondo, Satoru; E.A.Fischer*
JNC TN9400 2000-004, 38 Pages, 1999/05
An analytic thermophysical property model using general function forms is developed for a reactor safety analysis code, SIMMER-III. The function forms arc designed to represent correct behavior of properties of reactor-core materials over wide temperature ranges, especially for the thermal conductivity and the viscosity near the critical point. The most up-to-date and reliable sources for uranium dioxide, mixed-oxide fuel, stainless stee1, and sodium available at present are used to determine parameters in the proposed functions. This model is also designed to be consistent with a SIMMER-III model on thermodynamic properties and equations of state for reactor-corc materials.
Saito, Hioraki*; Iriya, Yoshikazu*
JNC TJ8440 99-003, 156 Pages, 1999/03
no abstracts in English
; Nishino, Yasuharu; ; Nakamura, Jinichi; *
JAERI-Tech 94-028, 147 Pages, 1994/11
no abstracts in English
Ugajin, Mitsuhiro
JAERI-M 92-065, 113 Pages, 1992/05
no abstracts in English
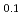 U
U O
O solid solution
solid solutionHinatsu, Yukio; Fujino, Takeo
Chemical Physics Letters, 172(2), p.131 - 136, 1990/08
Times Cited Count:8 Percentile:37.22(Chemistry, Physical)no abstracts in English
 U
U O
O (x
(x 0,x
0,x 0) and rhombohedral Eu
0) and rhombohedral Eu UO
UO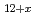 (x
(x 0)
0)Fujino, Takeo; Ouchi, Kinji; *; *; *
Journal of Nuclear Materials, 174, p.92 - 101, 1990/00
Times Cited Count:25 Percentile:89.09(Materials Science, Multidisciplinary)no abstracts in English
 U
U O
O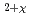 (M=Th, Zr, Ce, La, Y, Sc, Ca) solid solutions
(M=Th, Zr, Ce, La, Y, Sc, Ca) solid solutionsHinatsu, Yukio; Fujino, Takeo
J. Less-Common Met., 155, p.347 - 361, 1989/00
no abstracts in English
; ; ; ; ; ; *; *
Anal. Chem., 58(2), p.458 - 462, 1986/00
Times Cited Count:11 Percentile:54.69(Chemistry, Analytical)no abstracts in English
; ;
J.Less-Common Met., 121, p.631 - 636, 1986/00
Times Cited Count:13 Percentile:80.3(Chemistry, Physical)no abstracts in English
Aratono, Yasuyuki; Nakashima, Mikio; Saeki, Masakatsu; Tachikawa, Enzo
Nihon Genshiryoku Gakkai-Shi, 27(2), p.139 - 144, 1985/00
Times Cited Count:0 Percentile:0.02(Nuclear Science & Technology)no abstracts in English
Fujino, Takeo; ;
Analytica Chimica Acta, 147, p.423 - 428, 1983/00
Times Cited Count:1 Percentile:11.06(Chemistry, Analytical)no abstracts in English
 during reactor operation
during reactor operation; Nakashima, Mikio; Saeki, Masakatsu; Tachikawa, Enzo
Journal of Nuclear Materials, 114, p.234 - 241, 1983/00
Times Cited Count:3 Percentile:44.36(Materials Science, Multidisciplinary)no abstracts in English